
For a detailed table with instrument specifications, look here: Spreadsheet table view of all MIC equipment. Note: recharge rates are most up-to-date on iLabs and non-UC rates are 2X the UC rate. Contact us with questions or specific imaging needs.
For instrument scheduling: MIC iLabs page
– Ultra-sensitive, inverted laser scanning confocal with 1-photon and 2-photon imaging, with GaAsP detectors
– Airyscan-2 super-resolution technology with Multiplex Mode
– Becker-Hickl Time-correlated single photon counting fluorescence lifetime imaging
– FCS (coming soon)
– full incubation (heating & CO2)

– Ultra-sensitive, upright laser scanning confocal with 1-photon and 2-photon imaging, GaAsP detectors, 34-channel spectral imaging
– Airyscan super-resolution technology, up to 1.7-fold better resolution than standard scanning confocal microscopes
– Large motorized stage for combined electrophysiology and imaging
– 20X CLARITY objective in addition to standard objectives
 Zeiss LSM 880 NLO AxioExaminer with Coherent Chameleon MPX OPO (Neo)
Zeiss LSM 880 NLO AxioExaminer with Coherent Chameleon MPX OPO (Neo)– Ultra-sensitive, upright confocal imaging with large motorized fixed stage
– 2-photon imaging from 700-1300 nm
– Visible lasers: 405, 458, 488, 514, 561, 591, 633nm
 Zeiss LSM 780 AxioExaminer with Spectra-Physics Maitai (Rachel)
Zeiss LSM 780 AxioExaminer with Spectra-Physics Maitai (Rachel)– Ultra-sensitive, upright confocal featuring 2-photon imaging with GaAsP detectors
– 34-channel spectral system with imaging in the visible range from 400-700nm; visible lasers: 405, 488, 514, 561, 633nm
– Large fixed stage for combined electrophysiology and imaging
 Zeiss LSM 990 AxioObserver (Rodimus Prime)
Zeiss LSM 990 AxioObserver (Rodimus Prime)– Inverted laser scanning confocal
– Motorized XY-stage for tile scan and multi-position experiments
– Live cell imaging capabilities: full incubation chamber with temperature, CO2 control and heated stage
– Lasers: 405, 458, 488, 514, 561, 591, 633nm

Zeiss Axio Scan 7 Slide Scanner(Stay Puft)
-100-slide automated slide scanner for fluorescence or IHC
-Filter cubes for:
-Slide scanner service available

– 100-slide automated slide scanner for fluorescence or IHC
– Filter cubes for: DAPI, eGFP, Cy3, mPlum, Cy5
– Slide scanning service available
 Echo Revolve (Bender)
Echo Revolve (Bender)Brightfield and fluorescence microscope which can revolve to become either an upright microscope or an inverted microscope. It has 4x, 10x and 20x air objectives. It’s super easy to use, runs on an iPad, and can be used for checking your brightfield stains or fluorescence signals prior to slide scanning or confocal imaging!
 Nikon Widefield Epifluorescent Microscope (The Herzmark)
Nikon Widefield Epifluorescent Microscope (The Herzmark)– Inverted compound microscope with DIC optics
– Incubation chamber, motorized stage, and Nikon PerfectFocus
– Filters: DAPI, eGFP, Rhodamine, Cy5
 Zeiss AxioZoom v16 FL (Zoom)
Zeiss AxioZoom v16 FL (Zoom)– Macro scope with 16x motorized zoom, fluorescence, BF transmitted or reflected light
– Motorized XY-stage, ideal for multi-position or tiling
– Fluorescent illumination with the following filter cubes: DAPI, eCFP, eGFP, eYFP, and mCherry
 Leica M205 Fluorescent Stereoscope (Johnny-5)
Leica M205 Fluorescent Stereoscope (Johnny-5)– Fluorescent stereoscope ideal for screening and dissections
– Filter cubes: CFP, GFP, YFP, and DS-Red
– Not equipped with a camera – for screening only.
 Nikon Spinning Disk Confocal (Wall-E)
Nikon Spinning Disk Confocal (Wall-E)– Inverted Yokogawa spinning disk confocal microscope for live cell imaging
– Nikon PerfectFocus
– Lasers: 405, 488, 561, and 637nm
– Full incubation enclosure with CO2, humidity, and heating
– Motorized XY stage
 3i Spinning Disc Confocal with SLM (Enterprise)
3i Spinning Disc Confocal with SLM (Enterprise)– Upright spinning disk confocal microscope with spatial light modulator (SLM)
– Large platform, Sutter-motorized XY-stage for electrophysiology
– Ideal for zebrafish, Drosophila, and brain slice work with 1-photon or 2-photon holographic stimulation
 Nikon/Andor Spinning Disk Confocal (Max)
Nikon/Andor Spinning Disk Confocal (Max)– Inverted spinning disk confocal with a motorized XY-stage and Nikon PerfectFocus
– Full enclosure incubation chamber for humidity, CO2 and temperature control for live cell imaging.
– Andor Clara and Andor iXon cameras

TruLive 3D Imager
The Bruker Luxendo TruLive3D Imager is optimized for fast 3D multi-sample volume imaging of delicate live specimens in their native 3D environment. Thus, it is particularly well-suited for time-lapse and high-throughput imaging of 3D spheroids, organoids, 3D cell cultures, and small embryos. Using an optical concept, with dual-sided illumination and single-lens detection from below, enables fast acquisition speed, high-resolution imaging, and minimal shadowing effects.
 Zeiss LightSheet.Z1 (Morpheus)
Zeiss LightSheet.Z1 (Morpheus)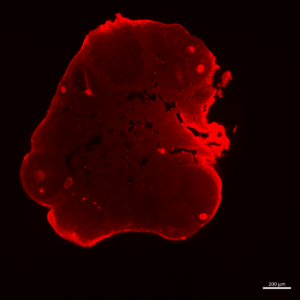
cleared ovary imaged in the lightsheet Z1
Selective Plane Illumination Microscopy (SPIM)

– Automated inverted microscope for live cell imaging
– Incubation chamber with CO2, temperature and humidity control
– Automated UV decontamination between sessions
– Combined confocal (LSM 900) and widefield microscope
– Objectives (air or water): 5x/0.35 air, 20x/0.7 air (2.2mm WD), 20x/0.95 air, 50x/1.2 water – all with 0.5x, 1x, and 2x tube lens magnification option (i.e., equivalent objective range from 2.5x up to 100x possible)
– Automatic water immersion for 50x water-dipping objective
– Lasers: 405, 488, 561, and 650 nm
– Widefield filters: 425/30 + 514/30 + 592/25 + 709/100, 467/24 + 555/25 + 687/145
– Brightfield: Differential interference contrast (DIC) and phase contrast microscopy
– Slides (up to 3), dishes (35 or 50mm), multi-well plates
– Airyscan 2 super resolution (140 nm resolution)
– Software: Zen Blue 3.2
This tutorial video is a must-watch before you come for training:
Learn more about Rocinante:
 DeepThought & Threadripper
DeepThought & Threadripper– Windows workstations with GPU processor and SSD hard drives for fast processing and rendering
– Software: Bitplane Imaris, Zeiss Zen, FIJI, Matlab, Adobe Suite
 Morpheus Processing Workstation
Morpheus Processing Workstation– Windows workstation with GPU processor for faster processing and rendering
– Software: arivis Vision4D, Zeiss Zen, FIJI
 Serenity Processing Workstation
Serenity Processing Workstation– Windows workstation processing lightsheet data
– Software: Zeiss Zen, FIJI
–>